|
A particular interest of the laboratory is protocadherin-15 (PCDH15). Arranged as a parallel dimer, it constitutes the lower third of the hair-cell tip link, binding at its N-terminus to cadherin-23 (CDH23), which as a parallel dimer constitutes the upper two thirds. At the membrane, PCDH15 binds to the mechanotransduction channel, TMC1, and to an accessory protein, LHFPL5. Mutations in PCDH15 cause the deafness and blindness of Usher syndrome type 1F.
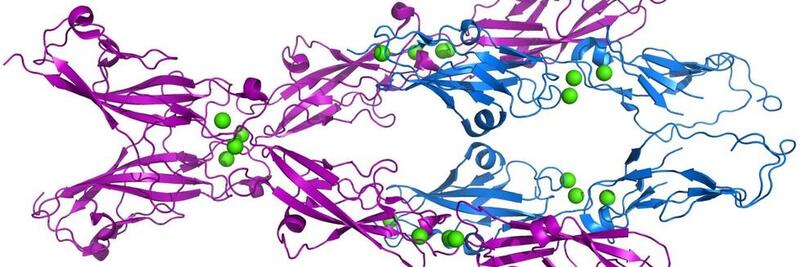
To understand the bond between PCDH15 and CDH23, we solved the X-ray structure of the bond between PCDH15 and CDH23, which comprises two extracellular cadherin (EC) repeats of PCDH15 and two EC repeats of CDH23. We used steered molecular dynamics simulations of the structure to understand the forces required to pull apart the single-stranded bond. This modeling was necessarily at short times and high forces. Later, Marcos Sotomayor in his own laboratory solved the tetrameric bond of the double-stranded tip link (below). |
|
|
|
|
X-ray crystal structure of the bond between PCDH15 (magenta) and CDH23 (blue). Two strands of PCDH15 bind to two strands of CDH23, with each pair forming an extended “handshake” bond. [Marcos Sotomayor] |
|
|
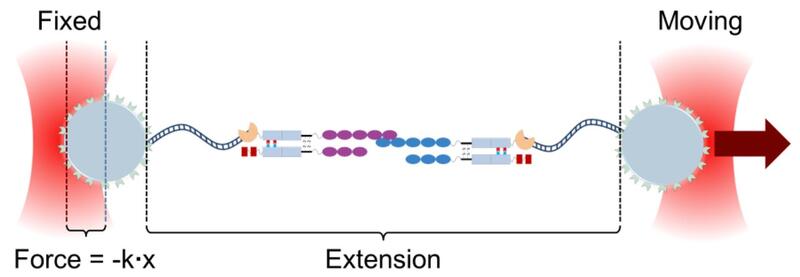
To measure the bond strength at more physiological forces and times, we synthesized either partial or full extracellular domains of PCDH15 and CDH23, dimerized them by fusion to antibody Fc domains, and coupled them to DNA strands by linking through a SNAP tag. The DNA strands were attached to glass beads that could be trapped and moved in a laser tweezers apparatus. We could then pull with increasing force until the bond broke, and measure the unbinding force under a variety of conditions. We found that the dimeric assembly of the cadherins substantially enhances the lifetime of a tip link: if one strand unbinds, it can quickly rebind before the other strand unbinds, extending the lifetime by tenfold or more at low force. The unbinding rate increases with force so that at high force the tip link quickly breaks, perhaps acting as a mechanical circuit breaker to protect the mechanotransduction complex. These measurements also suggest that the tip link is far more dynamic than previously believed. |
|
|
|
|
Schematic of the experimental arrangement. Extracellular domains of PCDH15 (magenta) and CDH23 (blue) were fused with Fc domains of antibodies (gray rectangles). The Fc domains were polarized to create specific dimers. Paired Fc domains were then linked to one end of a DNA tether through a SNAP tag. DNA tethers were biotinylated at the other end and linked to streptavidin-coated 3-μm silica microspheres, passivated with a polyethylene glycol brush. These beads, attached to either CDH23- or PCDH15-linked DNA constructs and distinguishable by a fluorescent marker, were placed in a flow cell on the microscope stage, and manipulated with a dual-beam optical tweezers setup. Beads were pulled apart until the bond ruptured, and the rupture force calculated from the calibrated force on the moving bead. |
|
|
PAPERS Sotomayor M, Weihofen WA, Gaudet R, Corey DP (2010) Structural determinants of cadherin-23 function in hearing and deafness. Neuron 66:85-100 Sotomayor M, Weihofen WA, Gaudet R, Corey DP (2012) Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492:128-32 Mulhall EM, Ward A, Yang D, Koussa MA, Corey DP, Wong WP (2021) Single-molecule force spectroscopy reveals the dynamic strength of the hair-cell tip-link connection. Nat Commun. 12:849. |